
That is to say, we weren’t looking at a general thermodynamic situation, but confined ourselves to studying a specific case with only one kind of molecule.

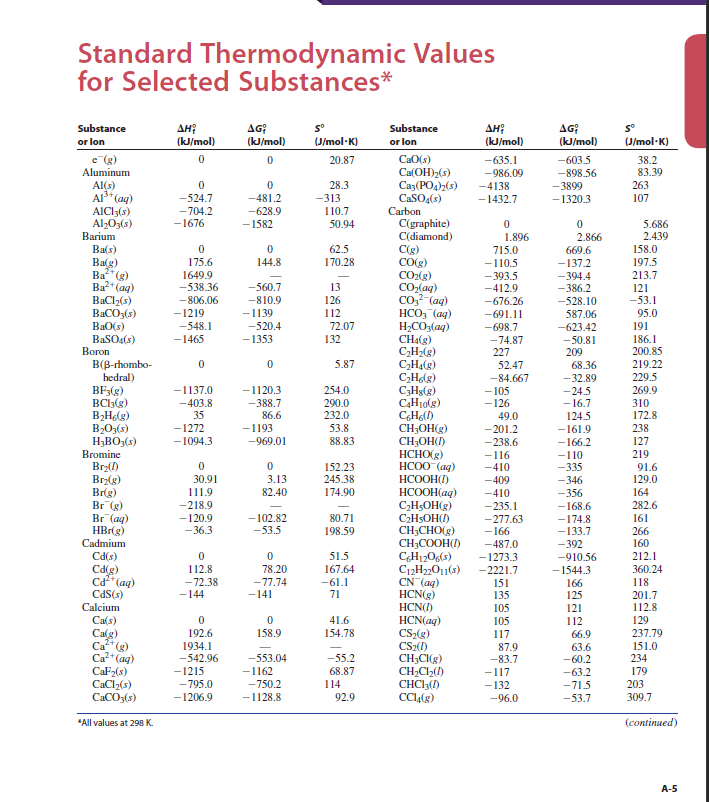
In the above case, we ended up with a specific enthalpy because we were doing a reaction for a specific substance, water. Oftentimes, enthalpy and specific enthalpy seem almost confusingly intertwined in such a way that it’s hard to know if one is using a general enthalpy or a specific one. It is essentially a value for the specific enthalpy change necessary to get one kilogram of water at boiling temperature (100 degrees Celsius) to change state to one kilogram of water vapor. In kilograms, this amounts to 2257 kJ/kg. From experimental data, this number is known to be 40.65 kJ/mol. This is known as the enthalpy of vaporization for water. Looking at the vaporization of water, the chemical equation is written as:įor a given mole of liquid water, there is a certain change in enthalpy that has to occur for that mole of water to change state to a gas. If a reaction adds energy to a system (endothermic), ΔH is positive and if a reaction subtracts energy from a system (exothermic), ΔH will be negative. This merely states that the total energy change after a reaction is equal to how much energy is present at the end subtracted by the amount we started with. In any open system, the following is true: We want to know what the change in enthalpy, ΔH, for a given reaction or process will be. In most thermodynamic applications, total enthalpy is not the quantity of interest. Where H is the total enthalpy, U is the energy of the work done in the system, p is pressure, and V is the volume of the system. This is to be seen as the specific enthalpy version of, and not to be confused with, the enthalpy equation:

Corrections to this equation are needed for real gasses, dependent on their molar weight and volume. Where u is the specific energy, p is the pressure and v is the volume. where P is pressure in Pascals, V is volume in Liters, n is number of moles, R is universal gas constant, T is temperature in Kelvin, k is the Boltzmann constant and N is the number of gas molecules.
Specific enthalpy can also be written in terms of specific energy, pressure, and specific volume such that the following equation is true: Where h is the specific enthalpy, H is the enthalpy of the system, and m is the total mass of the system. Specific enthalpy is calculated by taking the total enthalpy of the system and dividing it by the total mass of the system. The SI units for specific enthalpy are kJ/kg (kilojoules per kilogram). Specific enthalpy is used in thermodynamic equations when one wants to know the energy for a given single unit mass of a substance. Specific Enthalpy is the total energy in a system due to pressure and temperature per unit of mass in that system.


 0 kommentar(er)
0 kommentar(er)
